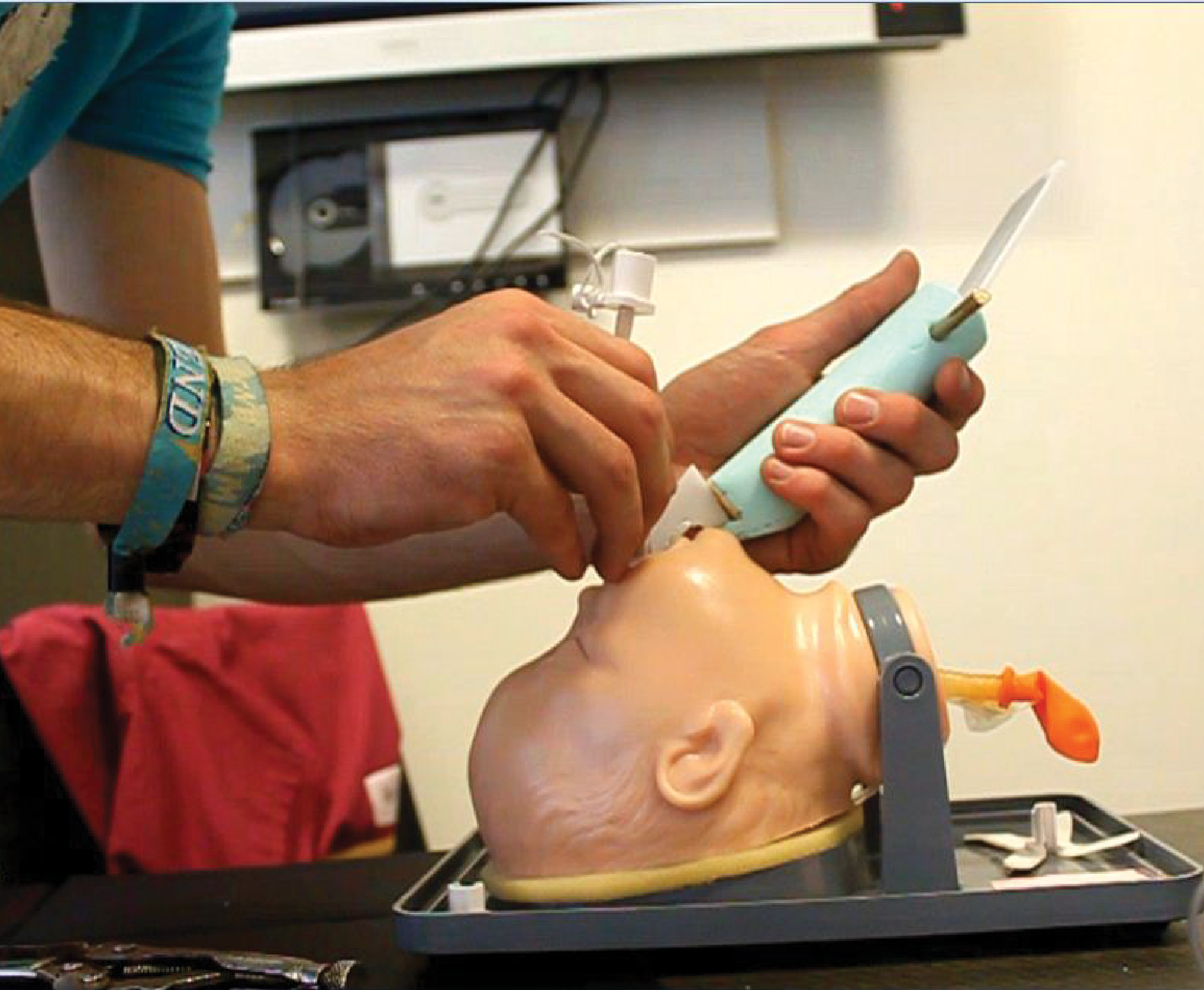
Design Challenge
Improve the direct method of tracheal intubation (placement of a plastic tube into the trachea to maintain an open airway), which is by direct line of sight, by using a video camera at the end of a specially designed blade. Develop a testing method to investigate the target patient, preterm infants. The need to test intubation on the most difficult airways was the selected method for proof of concept. This testing of blade designs took place in a clinical setting using healthcare professionals in Cork University Hospital (CUH).
Contextual Awareness
The target users that we investigated looked at normal airways and some of the most difficult airways to intubate. In <2% of cases tracheostomy would be preformed, because it was impossible to use the intubation method, and time was the main concern. Therefore the device itself must be quickly setup and have fail-safes that ensure it is always ready for use and never out of battery or missing parts.
Design Solution
Looking at the results from proof of concept round 1, in CUH, the clear winners were the simpler designs. These proved much easier to use, and could be held by right and left handed participants easy without any confusion. These were developed into high fidelity 3D printed models to test different blade attachment methods and packaging configurations, to reduce use errors and improve the overall safety for the patients by improve setup speed.
Improved Usability
To further reduce the setup time, it was advantageous to eliminate the need to attach the device to a screen. Therefore the use of wireless technology was seen to deliver increased outcomes for patients.
Direct vs Video Testing
Increased first-time intubation of a difficult airway by 17% for Video vs Direct method during clinical
usability testing in Cork University Hospital. This prototype was developed further by Tyndall National Research Institute and satisfied their internal audits for ISO 62366 for usability standards. It was also subject to ISO 14971 for risk management.
usability testing in Cork University Hospital. This prototype was developed further by Tyndall National Research Institute and satisfied their internal audits for ISO 62366 for usability standards. It was also subject to ISO 14971 for risk management.